Epigenetics: Feast, Famine, and Fatness
I have a simple Biology 101 question: How are biological traits passed down from one generation to the next?
Pretty easy, right? If you said “genetics” then you’re only half right.
The other half of the answer is surprising — so surprising, in fact, that if I’d proposed it during my undergrad years, I’d have been ridiculed as a member of the Flat Earth Society.
In the last five to ten years, there has been more and more evidence showing there is a non-genetic part that can be passed down to children and even grandchildren. As of this summer there are over 100 scientific articles documenting non-DNA inheritance, also called transgenerational epigenetics (1).
Non-DNA inheritance (aka epigenetics)
Non-DNA inheritance or epigenetics means that the sequence of the DNA doesn’t change, but access to the DNA changes biochemically. This altered DNA can then be passed down to children and grandchildren.
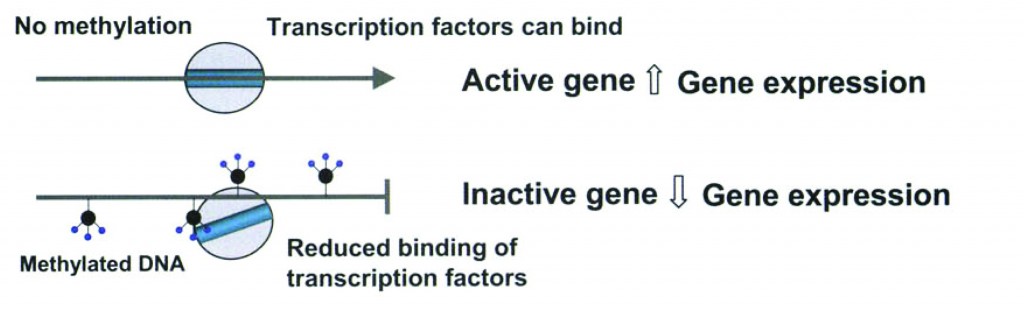
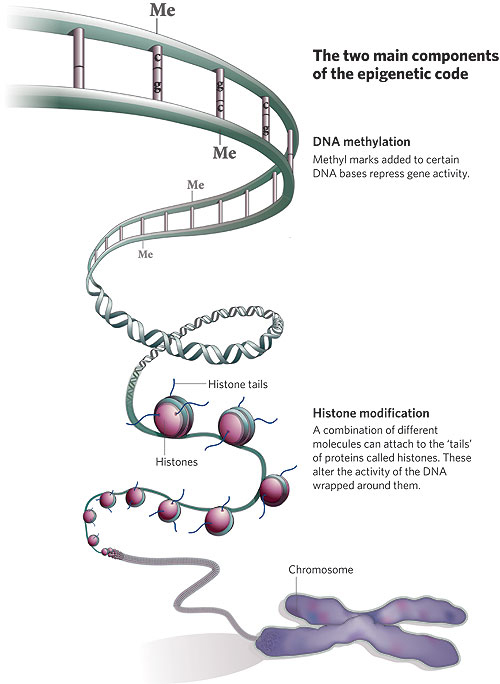
A brief molecular biology interlude: Access to DNA is changed by adding methyl groups to the DNA (take a look at Figure 1) or acetylating or deacetylating the histones the DNA is wrapped around (Figure 2).
For more info on histones and DNA access, read my last research review.
Figure 1: Methylation and gene expression. (2)
Figure 2: Two major components of epigenetics (3)
Think of the sequence of DNA as letters in a book. You can change the book’s story if you staple some of the pages together.
You didn’t change or delete a single letter in the book, but you can’t read certain pagers or an entire chapter, and that can change the entire story.
Think of methylation as a staple that stops a cell from reading genes.
What’s the big deal, DNA or non-DNA?
The big deal is that non-DNA inheritance responds to your environment. Things like diet can change your non-DNA inheritance.
The tale of two genetically identical mice
If you take a look at Figure 3 below, you see two mice that are, believe it or not, genetically identical (4).
They don’t look genetically identical — they don’t even look like the same species of mouse — but they are. They’re a breed of mouse called agouti.
Figure 3: Agouti mice
Agouti mice are usefully for epigenetic studies for two reasons:
- They have a gene (a genetically modified agouti gene) that makes them obese and yellow.
- The agouti gene can be turned off by methylation (or staples in our book analogy).
Mice with the agouti gene turned off are genetically identical to their yellow obese kin, but are skinny and brown, because of methylation of the agouti gene.
How is this possible? Nutrition.
The mother of the skinny brown mouse ate mouse chow supplemented with folic acid (vitamin B12), choline chloride and anhydrous betaine (all chemicals that donate methyl to DNA) for two weeks before mating, though the pregnancy (about 3 weeks) and lactation.
Once the mice were weaned they ate the same food until they were 21 days old – when the picture was taken.
What about humans?
To quote Alexander Pope, “The proper study of mankind is man”; thus in this week’s review I look at a study of epigenetics (non-DNA inheritance) in people.
Heijmans BT, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008 Nov 4;105(44):17046-9.
Methods
Boy during Dutch hunger winter
This study is a bit different then most studies because it uses a historical event (the Dutch hunger winter) as a way of studying non-DNA changes.
The Dutch hunger winter occurred from November 1944 to May 1945, when the Dutch in German-occupied Netherlands lived through a severe famine.
Daily rations during the famine:
- Started at less than 1000 kcal in November 1944
- Ended at around 500 kcal by April 1945
In May 1945 the Dutch were liberated, rationing stopped, the famine ended and everybody ate pretty much what they wanted (4).
Two things happened that makes this unfortunate event very useful scientifically:
- We have the health information, because registries and health care records were maintained and are still available.
- We know when the famine started and stopped, because implementation of rationing was sudden and the rations were well documented.
There were three groups for this study:
- People that were born or conceived during the famine (311 of them).
- Same-sex siblings that were born before or conceived after the famine (311 of them).
- Unrelated people as controls (349 of them).
Non-DNA changes in IGF2
For this study the researchers looked at the IGF2 gene. IGF2 is involved in human growth and development and it can be methylated.
The researchers compared how many methyl groups the IGF2 gene had in each of the three groups. More methylation means less IGF2.
Results
Six decades after being conceived during the Dutch hunger winter people had less methylation on their IGF2 gene compared to their unexposed same-sex brother or sister.
Other studies on the same group found that people conceived during the famine had impaired glucose tolerance, hypercholesterolaemia, raised blood pressure and higher rates of obesity in adulthood (6-8). However, researchers didn’t look at whether these differences were because of epigenetic changes or developmental problems caused by the famine.
Why would being conceived during a famine make you more likely to have metabolic and cardiovascular problems?
This study’s researchers think it’s because your body tries to become as efficient as possible at storing calories, since there isn’t much food around during your development. That works well, until suddenly you have all the food you need and more.
Once you have too many calories, or even simply enough calories, your body can’t stop storing calories. This causes you to have metabolic and cardiovascular problems associated with being overweight.
Conclusion
While famine is an extreme case of altered nutrition – all nutrients are scarce — it gives scientist a pretty useful tool to look at how nutrition can change our epigenetics.
This is the first study to show that the short-term environmental conditions at time of conception affects people’s epigenetics.
Not to over-dramatize, but epigenetics will be the next major biological frontier.
Don’t believe me?
What if I told you scientists found that whether or not your paternal grandfather had enough food as a teenager would change how long you lived, but only if you were male. Or that being born after your mother’s gastric bypass surgery is better for your health?
Same-sex grandparents and starvation
Swedish scientists found that if your grandfather or grandmother went through a famine as teenagers, you would have higher mortality risk ratios, but only if you were the same sex as the grandparent who starved.
Grandfather starvation only changed the grandson’s mortality risk and grandmother starvation only changed the granddaughter’s mortality risk (9). Wow.
Gastric bypass surgery and children’s obesity
Another study looked at mothers who had gastric bypass surgery and obesity in their children.
Do you think it would matter whether the children were born before or after the surgery? Since it was surgery and not a change in lifestyle, I would have guessed there would be no difference (10).
Possibly because of epigenetics, the study found that children born to women post-gastric bypass surgery were 52% less likely to be obese compared to their brothers and sisters born while their mother was obese. Wow, again.
Bottom line
We probably have more control over our DNA than we’ve been led to believe.
While you can’t change the sequence of your DNA, you may be able to change whether or not it is activated.
As it turns out, eating poorly and restrictively can have effects not just for you but your children as well.
"Zemliia" (Earth). (Commemorates the 1933 famine in Ukraine)
References
- Jablonka E, Raz G. Transgenerational epigenetic inheritance: prevalence,mechanisms, and implications for the study of heredity and evolution. Q Rev Biol.2009 Jun;84(2):131-76. Review.
- Ling C, Groop L. Epigenetics: a molecular link between environmental factors and type 2 diabetes. Diabetes. 2009 Dec;58(12):2718-25. Review.
- Qiu J. Epigenetics: unfinished symphony. Nature. 2006 May 11;441(7090):143-5.
- Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003 Aug;23(15):5293-300.
- Lumey LH, et al. Cohort profile: the Dutch Hunger Winter families study. Int J Epidemiol. 2007 Dec;36(6):1196-204.
- Roseboom TJ, et al. Coronary heart disease after prenatal exposure to the Dutch famine, 1944-45. Heart. 2000 Dec;84(6):595-8.
- Roseboom TJ, van der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Bleker OP. Plasma lipid profiles in adults after prenatal exposure to the Dutch famine. Am J Clin Nutr. 2000 Nov;72(5):1101-6.
- Painter RC, Roseboom TJ, Bleker OP. Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod Toxicol. 2005 Sep-Oct;20(3):345-52. Review.
- Pembrey ME, et al. ALSPAC Study Team. Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet. 2006 Feb;14(2):159-66.
- Kral JG, et al. Large maternal weight loss from obesity surgery prevents transmission of obesity to children who were followed for 2 to 18 years. Pediatrics. 2006. Dec;118(6):e1644-9.
Offering multiple perspectives from many fields of human inquiry that may move all of us toward a more integrated understanding of who we are as conscious beings.
Thursday, December 31, 2009
Precision Nutrition - Epigenetics: Feast, Famine, and Fatness
Great post from Helen Kollias writing at the Precision Nutrition blog. Understanding the science of fat accretion and fat loss is crucial, at least for those of us trying to help others.
Tags:
Subscribe to:
Post Comments (Atom)



No comments:
Post a Comment