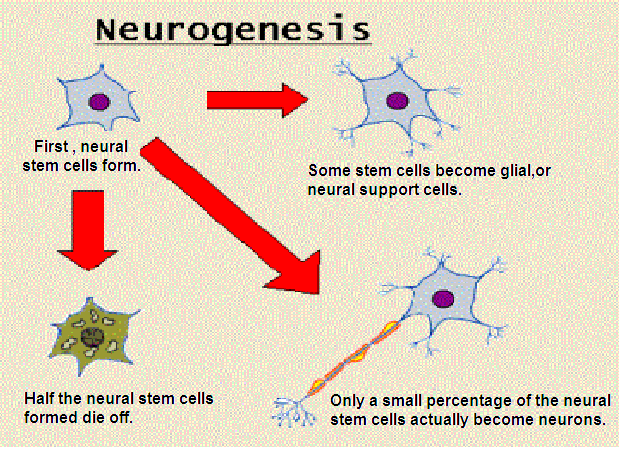
Interesting new research out of Duke University on how specific neurons tell neuronal stem cells where to go to repair damage. Below this press release is the abstract and citation from Nature Neuroscience (where the article is, of course, embargoed).
Neuron Tells Stem Cells to Grow New Neurons
Researchers identify first piece of new brain-repair circuit
June 2, 2014 | By Karl Leif Bates
 In this artist's representation of the adult subependymal neurogenic niche (viewed from underneath the ependyma), electrical signals generated by the ChAT+ neuron give rise to newborn migrating neuroblasts, seen moving over the underside of ependymal cells. Illustration by O’Reilly Science Art.
In this artist's representation of the adult subependymal neurogenic niche (viewed from underneath the ependyma), electrical signals generated by the ChAT+ neuron give rise to newborn migrating neuroblasts, seen moving over the underside of ependymal cells. Illustration by O’Reilly Science Art.
Durham, NC - Duke researchers have found a new type of neuron in the adult brain that is capable of telling stem cells to make more new neurons. Though the experiments are in their early stages, the finding opens the tantalizing possibility that the brain may be able to repair itself from within.
Neuroscientists have suspected for some time that the brain has some capacity to direct the manufacturing of new neurons, but it was difficult to determine where these instructions are coming from, explains Chay Kuo, M.D. Ph.D., an assistant professor of cell biology, neurobiology and pediatrics.
In a study with mice, his team found a previously unknown population of neurons within the subventricular zone (SVZ) neurogenic niche of the adult brain, adjacent to the striatum. These neurons expressed the choline acetyltransferase (ChAT) enzyme, which is required to make the neurotransmitter acetylcholine. With optogenetic tools that allowed the team to tune the firing frequency of these ChAT+ neurons up and down with laser light, they were able to see clear changes in neural stem cell proliferation in the brain.
The findings appeared as an advance online publication June 1 in the journal Nature Neuroscience.
The mature ChAT+ neuron population is just one part of an undescribed neural circuit that apparently talks to stem cells and tells them to increase new neuron production, Kuo said. Researchers don't know all the parts of the circuit yet, nor the code it's using, but by controlling ChAT+ neurons' signals Kuo and his Duke colleagues have established that these neurons are necessary and sufficient to control the production of new neurons from the SVZ niche.
"We have been working to determine how neurogenesis is sustained in the adult brain. It is very unexpected and exciting to uncover this hidden gateway, a neural circuit that can directly instruct the stem cells to make more immature neurons," said Kuo, who is also the George W. Brumley, Jr. M.D. assistant professor of developmental biology and a member of the Duke Institute for Brain Sciences. "It has been this fascinating treasure hunt that appeared to dead-end on multiple occasions!"
Kuo said this project was initiated more than five years ago when lead author Patricia Paez-Gonzalez, a postdoctoral fellow, came across neuronal processes contacting neural stem cells while studying how the SVZ niche was assembled.
The young neurons produced by these signals were destined for the olfactory bulb in rodents, as the mouse has a large amount of its brain devoted to process the sense of smell and needs these new neurons to support learning. But in humans, with a much less impressive olfactory bulb, Kuo said it's possible new neurons are produced for other brain regions. One such region may be the striatum, which mediates motor and cognitive controls between the cortex and the complex basal ganglia.
"The brain gives up prime real estate around the lateral ventricles for the SVZ niche housing these stem cells," Kuo said. "Is it some kind of factory taking orders?" Postdoctoral fellow Brent Asrican made a key observation that orders from the novel ChAT+ neurons were heard clearly by SVZ stem cells.
Studies of stroke injury in rodents have noted SVZ cells apparently migrating into the neighboring striatum. And just last month in the journal Cell, a Swedish team observed newly made control neurons called interneurons in the human striatum for the first time. They reported that interestingly in Huntington's disease patients, this area seems to lack the newborn interneurons.
"This is a very important and relevant cell population that is controlling those stem cells," said Sally Temple, director of the Neural Stem Cell Institute of Rensselaer, NY, who was not involved in this research. "It's really interesting to see how innervations are coming into play now in the subventricular zone."
Kuo's team found this system by following cholinergic signaling, but other groups are arriving in the same niche by following dopaminergic and serotonergic signals, Temple said. "It's a really hot area because it's a beautiful stem cell niche to study. It's this gorgeous niche where you can observe cell-to-cell interactions."
These emerging threads have Kuo hopeful researchers will eventually be able to find the way to "engage certain circuits of the brain to lead to a hardware upgrade. Wouldn't it be nice if you could upgrade the brain hardware to keep up with the new software?" He said perhaps there will be a way to combine behavioral therapy and stem cell treatments after a brain injury to rebuild some of the damage.
The questions ahead are both upstream from the new ChAT+ neurons and downstream, Kuo says. Upstream, what brain signals tell ChAT+ neurons to start asking the stem cells for more young neurons? Downstream, what's the logic governing the response of the stem cells to different frequencies of ChAT+ electrical activity?
There's also the big issue of somehow being able to introduce new components into an existing neuronal circuit, a practice that parts of the brain might normally resist. "I think that some neural circuits welcome new members, and some don't," Kuo said.
In addition to Paez-Gonzalez, Asrican, and Kuo, Erica Rodriguez, a graduate student in the neurobiology training program, is also an author. This research was supported by the National Institutes of Health, David & Lucile Packard Foundation, and George Brumley Jr. Endowment.
More Information
Contact: Karl Leif Bates
Phone: (919) 681-8054
Email: karl.bates@duke.edu © 2014 Office of News & Communications
615 Chapel Drive, Box 90563, Durham, NC 27708-0563 | (919) 684-2823
* * * * *
Full Citation:
Paez-Gonzalez, P, Asrican, B, Rodriguez, E, and Kuo, CT, (2014, June 1). Identification of distinct ChAT+ neurons and activity-dependent control of postnatal SVZ neurogenesis. Nature Neuroscience; doi:10.1038/nn.3734 - ePub ahead of print
Identification of distinct ChAT+ neurons and activity-dependent control of postnatal SVZ neurogenesis
Patricia Paez-Gonzalez, Brent Asrican, Erica Rodriguez & Chay T Kuo
Abstract
Postnatal and adult subventricular zone (SVZ) neurogenesis is believed to be primarily controlled by neural stem cell (NSC)-intrinsic mechanisms, interacting with extracellular and niche-driven cues. Although behavioral experiments and disease states have suggested possibilities for higher level inputs, it is unknown whether neural activity patterns from discrete circuits can directly regulate SVZ neurogenesis. We identified a previously unknown population of choline acetyltransferase (ChAT)+ neurons residing in the rodent SVZ neurogenic niche. These neurons showed morphological and functional differences from neighboring striatal counterparts and released acetylcholine locally in an activity-dependent fashion. Optogenetic inhibition and stimulation of subependymal ChAT+ neurons in vivo indicated that they were necessary and sufficient to control neurogenic proliferation. Furthermore, whole-cell recordings and biochemical experiments revealed direct SVZ NSC responses to local acetylcholine release, synergizing with fibroblast growth factor receptor activation to increase neuroblast production. These results reveal an unknown gateway connecting SVZ neurogenesis to neuronal activity-dependent control and suggest possibilities for modulating neuroregenerative capacities in health and disease.
No comments:
Post a Comment