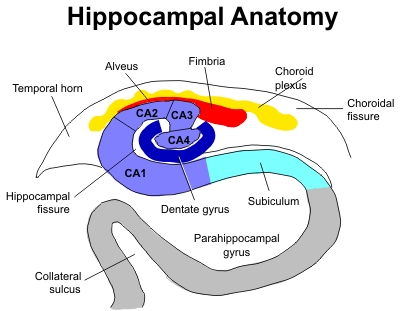
The first of these two articles looks at the history and current state of our knowledge of neurogenesis in the adult hippocampus. The second article reveals research suggesting the social isolation inhibits hippocampal neurogenesis.
From Wikipedia:
Neurogenesis (birth of neurons) is the process by which neurons are generated from neural stem cells and progenitor cells. Most active during pre-natal development, neurogenesis is responsible for populating the growing brain with neurons. Recently neurogenesis was shown to continue in several small parts of the brain of mammals: the hippocampus and the subventricular zone. Studies have indicated that the hormone testosterone in vertebrates, and the prohormone ecdysone in insects, have an influence on the rate of neurogenesis.The first article is a briefer review piece, so it is included here in its entirety. The second article is much longer and only the abstract and introduction are included.
Functional neurogenesis in the adult hippocampus: Then and now
Krishna C. Vadodaria [1,2] and Sebastian Jessberger [1,2]
1. Faculty of Medicine and Science, Brain Research Institute, University of Zurich, Zurich, Switzerland
2. Neuroscience Center Zurich, University of Zurich and ETH Zurich, Zurich, Switzerland
Introduction
After two decades of research, the neurosciences have come a long way from accepting that neural stem/progenitor cells (NSPCs) generate new neurons in the adult mammalian hippocampus to unraveling the functional role of adult-born neurons in cognition and emotional control. The finding that new neurons are born and become integrated into a mature circuitry throughout life has challenged and subsequently reshaped our understanding of neural plasticity in the adult mammalian brain. It is now widely accepted that neurogenesis in the adult central nervous system occurs in multiple brain regions within the rodent brain, including the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus (DG). Since the discovery of ongoing neurogenesis in the adult brain, the field has been addressing questions regarding the cellular identity of adult NSPCs, the molecular pathways regulating maturation and integration of newborn neurons into preexisting circuitries, and how new neurons contribute to adult brain function. Technological advances over the last two decades such as targeted modulation (loss- and gain-of-function) of adult neurogenesis and refinements in behavioral testing paradigms have enabled us to begin addressing these questions directly. Here we give a brief overview of old and new studies examining the function of adult hippocampal neurogenesis (AHN) in the context of evolving technology, which has exponentially expanded our understanding of the neurogenic process in the adult mammalian brain.
Early Studies: From Correlation to Causality
Early on, the field went through a phase of correlating levels of AHN with performance in behavioral tests of hippocampus-dependent learning and memory, and affective behavior. Manipulations that increase AHN such as environmental enrichment, physical activity, and also treatment with certain antidepressants were found to enhance performance in spatial navigation tasks (e.g., Morris water maze; MWM) and in tests of anxiety-related behaviors (forced swim test, elevated plus maze) (Kim et al., 2012). Conversely, stress, aging, and inflammation, all of which negatively affect AHN, resulted in decreased performance in tasks of spatial navigation and emotion-related behaviors (Kim et al., 2012). Although correlative, these data generated in the late 1990s and early 2000s, suggested a role for AHN in hippocampus-dependent processes of cognition and emotion. The first studies attempting to show causal relationship between AHN and hippocampus-dependent behavior were published in the early 2000s, using the antimitotic drug methylazoxymethanol acetate (MAM) and focal irradiation of the hippocampus to ablate AHN. MAM-treated and focally irradiated mice showed impairments in hippocampus-dependent trace-conditioning and certain forms of long-term spatial memory (Shors et al., 2001; Snyder et al., 2005; Deng et al., 2009), suggesting that AHN was required for particular aspects of learning and memory. However, seemingly inconsistent findings from multiple studies with confounding variables such as incomplete elimination of neurogenesis and unwanted off-target effects (such as irradiation-induced inflammation) impeded a precise understanding of the contribution of AHN to hippocampal function (Deng et al., 2010).
Functional Hippocampal Neurogenesis and Evolving Methodology
Significant advances in conditional gene targeting allowing the generation of transgenic mice and virus-based approaches enabled the selective targeting of adult hippocampal NSPCs and their neuronal progeny, and revealed not only the molecular pathways important for the different stages of neurogenesis, but also specific behavioral correlates of altered AHN (Saxe et al., 2006; Dupret et al., 2008; Jessberger et al., 2009; Deng et al., 2010; Ming and Song, 2011). Commonly used approaches include the expression of cell death-inducing genes (such as diphtheria toxin or its receptor and thymidine kinase that kills dividing cells upon gancyclovir injections), overexpression of pro-apoptotic genes (such as Bax), and expression of light-sensitive ion channels (such as channelrhodopsins enabling conditional depolarization or hyperpolarization of newborn neurons) in NSPCs and/or their neuronal progeny (Deng et al., 2010). Fewer methods have been utilized to genetically boost neurogenesis. One elegant approach has been to utilize transgenic mice where the pro-apoptotic gene BAX was conditionally deleted in nestin-expressing NSPCs (iBAXnestin), resulting in substantially enhanced levels of AHN (Sahay et al., 2011a). As compared to previous cytostatic drug- and irradiation-based strategies, these techniques improved temporal and tissue-specific control for ablating the desired neuronal population. Studies utilizing these strategies in combination with an array of behavioral tests have revealed novel roles for AHN. Together with correlational studies, genetic, and pharmacological approaches to manipulate levels of AHN are currently being used to understand the functional significance of AHN. Spatial discrimination tasks such as feared context, radial-arm maze, modified MWM, and the two-choice discrimination task have been utilized to test for a function of newborn neurons (Saxe et al., 2007; Clelland et al., 2009; Deng et al., 2009; Sahay et al., 2011a; Nakashiba et al., 2012) and there is now sufficient evidence suggesting that AHN plays a crucial role in the pattern separation functions of the DG (Treves et al., 2008; Yassa and Stark, 2011). The two-choice discrimination task where mice must discriminate between spatially proximate stimuli may become one of the behavioral tasks of choice (Clelland et al., 2009; Mctighe et al., 2009). Complementary approaches to knockdown AHN revealed selective deficits in this task and the radial arm maze. On the other hand, boosting AHN by genetically enhancing newborn neuron survival (using iBaxnestin) enhances discrimination between similar contexts in a contextual fear-conditioning task (Sahay et al., 2011a). Notably, AHN becomes critical only when contexts/patterns become more similar and therefore more difficult to discriminate during recall; thus, AHN seems to be dispensable for discriminating between highly dissimilar contexts/patterns but crucial for computing and discerning highly similar input patterns. Transgenic strategies enabling selective ablation of young and adult-born DG neurons vs. mature DG granule neurons in combination with modifications of the MWM show that in the absence of mature neurons, separation between similar spatial contexts is enhanced, whereas, “completing” a pattern with only a subset of the cues is impaired (Nakashiba et al., 2012). These results highlight an interesting interplay between “newborn” and “old” neuronal populations, suggesting different yet complementary functions of pattern “separation” vs. “completion,” respectively. Collectively, studies from multiple labs provide evidence of a strong link between AHN and proposed pattern separation functions of the DG (Sahay et al., 2011b). Furthermore, recent data using novel transgenic mice and virus-based approaches (e.g., optogenetics and TK-based approaches) support the hypothesis that new neurons are particularly important for memory encoding and retrieval during a critical period 4–8 weeks after new neurons are born (Deng et al., 2009; Gu et al., 2012).
Recent reports also support a role for AHN in emotional control and affective behavior. These studies benefitted not only from novel methods to ablate AHN, but also refinements in testing paradigms for specific aspects of emotion-related behaviors (Samuels and Hen, 2011; Kheirbek et al., 2012). Particularly, irradiated and transgenic mice with diminished AHN exhibit signs of heightened stress response as observed in the food avoidance test (after acute stress), increased despair-like behavior in the forced swim test, and increased anhedonia in sucrose preference tests (Snyder et al., 2011). These deficits may be in part due to a dysfunctional regulation of the hypothalamic-pituitary-adrenal (HPA) axis that may lead to a disproportionate response to stress-inducing stimuli in mice with impaired AHN (Snyder et al., 2011). Interestingly, although ablation of AHN led to a heightened stress response along with behavioral correlates of depression-like behaviors, increasing neurogenesis by itself does not appear to be sufficient for promoting anxiolytic or antidepressant-like behaviors in the iBax mice (Sahay et al., 2011a). However, this may be due to a “ceiling” effect or due to limitations of current testing paradigms for examining “gain of function” in emotion-related behaviors.
Functional AHN: Open Questions
Accumulating evidence over the last years has clearly demonstrated a role for AHN in hippocampus-dependent cognition and emotional control. However, it is currently unclear how exactly newborn neurons shape the DG circuitry and mediate DG-dependent pattern separation. A large number of open questions remain: how are individual patterns represented in the DG (Deng et al., 2013)? How does the hippocampal circuit “change” with the addition of each pattern-associated cohort of newborn neurons? How does top-down or cortical input regulate AHN and its function in learning new information? How much do newborn young neurons contribute to memory engrams in the DG? How do adult-born hippocampal neurons regulate the HPA axis, which contributes to the neurogenesis-associated regulation of anxiety-related behaviors? Do distinct subsets of newborn neurons contribute to pattern separation vs. emotional regulation role of the DG? Other questions pertain to the relevance of varying levels of AHN, basally, by environmental stimuli, and in the context of disease: How do variations in AHN contribute to individuality in exploratory behavior and could this be extended to humans (Freund et al., 2013)? How does aging regulate AHN and can boosting AHN alleviate age-related decline in aspects of cognition? Can AHN be harnessed for endogenous brain repair and restoration of neuronal function in diseases that is associated with diminished or altered AHN, such as major depression, epilepsy, Alzheimer's disease, and Parkinson's disease? Interestingly, recent findings that levels of hippocampal neurogenesis remain substantial even through the fifth decade of life in the adult human brain, opens up possibilities for doing functional studies in humans related to AHN (Spalding et al., 2013), for example by combining non-invasive imaging strategies together with DG-dependent behavioral paradigms (Brickman et al., 2011; Yassa and Stark, 2011; Dery et al., 2013). With the development of novel genetic tools there is great hope for answering these questions, however, it also seems plausible that we need to develop more refined and sensitive testing paradigms to closely examine AHN-dependent behaviors. In addition, it is clear that most genetic approaches are only suitable for studies using mice, limiting the possibility to use different species to broaden the relevance of the obtained findings. Thus, developing novel methods to measure and /or manipulate AHN in primates and even humans will be important to move the field toward biomedical relevance.
Be that as it may, the finding that the adult mammalian brain continuously generates new neurons throughout life has contributed significantly to our understanding of brain functioning and recent technological advances provide further impetus for studying the function of AHN in health and disease.
Acknowledgments
Krishna C. Vadodaria is currently supported by a postdoctoral fellowship of the Swiss National Science Foundation (SNSF). We apologize to all authors whose work is not cited due to space constraints.
References
Brickman, A. M., Stern, Y., and Small, S. A. (2011). Hippocampal subregions differentially associate with standardized memory tests. Hippocampus 21, 923–928. doi: 10.1002/hipo.20840 Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Clelland, C. D., Choi, M., Romberg, C., Clemenson, G. D. Jr., Fragniere, A., Tyers, P., et al. (2009). A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325, 210–213. doi: 10.1126/science.1173215 Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Deng, W., Aimone, J. B., and Gage, F. H. (2010). New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 11, 339–350. doi: 10.1038/nrn2822 Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Deng, W., Mayford, M., and Gage, F. H. (2013). Selection of distinct populations of dentate granule cells in response to inputs as a mechanism for pattern separation in mice. Elife 2:e00312. doi: 10.7554/eLife.00312 Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Deng, W., Saxe, M. D., Gallina, I. S., and Gage, F. H. (2009). Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J. Neurosci. 29, 13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009 Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Dery, N., Pilgrim, M., Gibala, M., Gillen, J., Wojtowicz, J. M., Macqueen, G., et al. (2013). Adult hippocampal neurogenesis reduces memory interference in humans: opposing effects of aerobic exercise and depression. Front. Neurosci. 7:66. doi: 10.3389/fnins.2013.00066 Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Dupret, D., Revest, J. M., Koehl, M., Ichas, F., De Giorgi, F., Costet, P., et al. (2008). Spatial relational memory requires hippocampal adult neurogenesis. PLoS ONE 3:e1959. doi: 10.1371/journal.pone.0001959 Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Freund, J., Brandmaier, A. M., Lewejohann, L., Kirste, I., Kritzler, M., Kruger, A., et al. (2013). Emergence of individuality in genetically identical mice. Science 340, 756–759. doi: 10.1126/science.1235294 Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Gu, Y., Arruda-Carvalho, M., Wang, J., Janoschka, S. R., Josselyn, S. A., Frankland, P. W., et al. (2012). Optical controlling reveals time-dependent roles for adult-born dentate granule cells. Nat. Neurosci. 15, 1700–1706. doi: 10.1038/nn.3260 Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Jessberger, S., Clark, R. E., Broadbent, N. J., Clemenson, G. D. Jr., Consiglio, A., Lie, D. C., et al. (2009). Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn. Mem. 16, 147–154. doi: 10.1101/lm.1172609 Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Kheirbek, M. A., Klemenhagen, K. C., Sahay, A., and Hen, R. (2012). Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat. Neurosci. 15, 1613–1620. doi: 10.1038/nn.3262 Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Kim, W. R., Christian, K., Ming, G. L., and Song, H. (2012). Time-dependent involvement of adult-born dentate granule cells in behavior. Behav. Brain Res. 227, 470–479. doi: 10.1016/j.bbr.2011.07.012 Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Mctighe, S. M., Mar, A. C., Romberg, C., Bussey, T. J., and Saksida, L. M. (2009). A new touchscreen test of pattern separation: effect of hippocampal lesions. Neuroreport 20, 881–885. doi: 10.1097/WNR.0b013e32832c5eb2 Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Ming, G. L., and Song, H. (2011). Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70, 687–702. doi: 10.1016/j.neuron.2011.05.001 Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Nakashiba, T., Cushman, J. D., Pelkey, K. A., Renaudineau, S., Buhl, D. L., Mchugh, T. J., et al. (2012). Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell 149, 188–201. doi: 10.1016/j.cell.2012.01.046 Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Sahay, A., Scobie, K. N., Hill, A. S., O'Carroll, C. M., Kheirbek, M. A., Burghardt, N. S., et al. (2011a). Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472, 466–470. doi: 10.1038/nature09817 Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Sahay, A., Wilson, D. A., and Hen, R. (2011b). Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron 70, 582–588. doi: 10.1016/j.neuron.2011.05.012 Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Samuels, B. A., and Hen, R. (2011). Neurogenesis and affective disorders. Eur. J. Neurosci. 33, 1152–1159. doi: 10.1111/j.1460-9568.2011.07614.x Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Saxe, M. D., Battaglia, F., Wang, J. W., Malleret, G., David, D. J., Monckton, J. E., et al. (2006). Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl. Acad. Sci. U.S.A. 103, 17501–17506. doi: 10.1073/pnas.0607207103 Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Saxe, M. D., Malleret, G., Vronskaya, S., Mendez, I., Garcia, A. D., Sofroniew, M. V., et al. (2007). Paradoxical influence of hippocampal neurogenesis on working memory. Proc. Natl. Acad. Sci. U.S.A. 104, 4642–4646. doi: 10.1073/pnas.0611718104 Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Shors, T. J., Miesegaes, G., Beylin, A., Zhao, M., Rydel, T., and Gould, E. (2001). Neurogenesis in the adult is involved in the formation of trace memories. Nature 410, 372–376. doi: 10.1038/35066584 Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Snyder, J. S., Hong, N. S., Mcdonald, R. J., and Wojtowicz, J. M. (2005). A role for adult neurogenesis in spatial long-term memory. Neuroscience 130, 843–852. doi: 10.1016/j.neuroscience.2004.10.009 Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Snyder, J. S., Soumier, A., Brewer, M., Pickel, J., and Cameron, H. A. (2011). Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476, 458–461. doi: 10.1038/nature10287 Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Spalding, K. L., Bergmann, O., Alkass, K., Bernard, S., Salehpour, M., Huttner, H. B., et al. (2013). Dynamics of hippocampal neurogenesis in adult humans. Cell 153, 1219–1227. doi: 10.1016/j.cell.2013.05.002 Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Treves, A., Tashiro, A., Witter, M. P., and Moser, E. I. (2008). What is the mammalian dentate gyrus good for? Neuroscience 154, 1155–1172. doi: 10.1016/j.neuroscience.2008.04.073 Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Yassa, M. A., and Stark, C. E. (2011). Pattern separation in the hippocampus. Trends Neurosci. 34, 515–525. doi: 10.1016/j.tins.2011.06.006 Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
* * * * *
Social isolation disrupts hippocampal neurogenesis in young non-human primates
Simone M. Cinini [1], Gabriela F. Barnabe [1], Nicole Galvão-Coelho [2], Magda A. de Medeiros [3], Patrícia Perez-Mendes [1], Maria B. C. Sousa [2], Luciene Covolan [1] and Luiz E. Mello [1]
1. Departamento de Fisiologia, Universidade Federal de São Paulo, São Paulo, BrazilSocial relationships are crucial for the development and maintenance of normal behavior in non-human primates. Animals that are raised in isolation develop abnormal patterns of behavior that persist even when they are later reunited with their parents. In rodents, social isolation is a stressful event and is associated with a decrease in hippocampal neurogenesis but considerably less is known about the effects of social isolation in non-human primates during the transition from adolescence to adulthood. To investigate how social isolation affects young marmosets, these were isolated from other members of the colony for 1 or 3 weeks and evaluated for alterations in their behavior and hippocampal cell proliferation. We found that anxiety-related behaviors like scent-marking and locomotor activity increased after social isolation when compared to baseline levels. In agreement, grooming—an indicative of attenuation of tension—was reduced among isolated marmosets. These results were consistent with increased cortisol levels after 1 and 3 weeks of isolation. After social isolation (1 or 3 weeks), reduced proliferation of neural cells in the subgranular zone of dentate granule cell layer was identified and a smaller proportion of BrdU-positive cells underwent neuronal fate (doublecortin labeling). Our data is consistent with the notion that social deprivation during the transition from adolescence to adulthood leads to stress and produces anxiety-like behaviors that in turn might affect neurogenesis and contribute to the deleterious consequences of prolonged stressful conditions.
2. Departamento de Fisiologia, Universidade Federal do Rio Grande do Norte, Natal, Brazil
3. Departamento de Ciências Fisiológicas, Universidade Federal Rural do Rio de Janeiro, Seropédica, Rio de Janeiro Brazil
Introduction
Read the whole article.
In the adult hippocampus, progenitor cells in the subgranular zone of the dentate gyrus give rise to new neurons that migrate into the granule cell layer, differentiate into granular neurons, and are capable of functional integration into the hippocampal circuitry (Gould and Gross, 2002; Van Praag et al., 2002; Kee et al., 2007). The functional role of hippocampal neurogenesis has not been fully understood until now, but despite the divergent results from different laboratories and models, most data points toward its involvement with specific aspects of learning, conditioning, and spatial information (for review see Balu and Lucki, 2009).
Reduction in hippocampal neurogenesis is associated with stress (Gould et al., 1998) mainly by means of increased excitatory transmission (Gould et al., 1997; Abraham et al., 1998), pro-inflammatory cytokines (Koo and Duman, 2008), diminished neurotrophins expression (Duman and Monteggia, 2006; Jacobsen and Mork, 2006), and glucocorticoid signaling (Wong and Herbert, 2005, 2006). Social isolation is a form of stress, which affects some hippocampal-related functions such as learning and memory and may lead to affective disorders. In marmosets there is a strong exponential negative correlation between the number of dentate proliferating cells and aging where 2–3 years-old animals are considered young adults, from 4 to 7 years they are middle-aged and above 8 years old they are considered old (Bunk et al., 2011). In the present study we used social isolation of young animals as the stressful event (Laudenslager et al., 1995; Stranahan et al., 2006) in order to characterize behavioral consequences of social isolation during the transition phase from adolescence to adulthood, when the animals are at the peak of dentate neurogenesis, so any disturbance might bear a greater relevance in the onset of future mood disorders.
The long-term effects of social isolation among rodent pups include decreased hippocampal neurogenesis, which can culminate in a reduced ability to cope with stressful events in adulthood (Laudenslager et al., 1995; Mirescu et al., 2004; Karten et al., 2005; Stranahan et al., 2006; Rizzi et al., 2007). As compared to rodents, social interactions in primates are considerably more important for the appropriate neuropsychological development (Rosenblum and Andrews, 1994). Marmosets partially deprived of parental care during infancy develop abnormal patterns of behavior that persist even when they are later reunited with their parents (Dettling et al., 2002a,b). In spite of the well-characterized behavioral consequences of social isolation during infancy in these animals, little is known about the neurobiological effects of social isolation during its transition to adulthood. In the present study we investigate the consequences of social isolation in the behavior and hippocampal neurogenesis in these non-human primates.
No comments:
Post a Comment