Here is the conclusion from the study:
This is a great overview - beyond me - and you can read the original article at PLoS ONE.Conclusions
Our data suggest that in the adult human hippocampus neurogenesis-associated features that have been identified in rodents show patterns, as well as qualitative and quantitative age-related changes, that are similar to the course of adult hippocampal neurogenesis in rodents. Consequently, although further validation as well as the application of independent methodology (e.g. electron microscopy and cell culture work) is desirable, our data will help to devise the framework for specific research on cellular plasticity in the aging human hippocampus.
Adult neurogenesis in humans: Murine Features of Neurogenesis in the Human Hippocampus
Jason Snyder | 02/04/2010Studies of adult neurogenesis often begin with the following sentence: “Adult neurogenesis occurs in all mammals examined, including humans.” More detail-oriented papers might say, “Adult neurogenesis occurs in all mammals examined, including humans…but not bats.” Here, the similarities between bats and humans become more evident than one might expect: it could be an equally long time before we understand adult neurogenesis in either of these species. Bats are (relatively) easy enough to study experimentally, but how many studies will be required to understand why neurogenesis does not occur in the adult bat brain? With humans, we have the opposite problem: the one study in humans that used the unambiguous cell-birth marker, BrdU, found adult neurogenesis. The second study may never exist.
Since the original Eriksson study, a number of studies have attempted to characterize adult neurogenesis in the human hippocampus, by immunostaining for endogenous markers of proliferating precursors and immature neurons, thereby getting around the inconvenient fact that most human brains do not contain BrdU (similar techniques have been used to characterize neurogenesis in wild animals). The problem is that the histology in human studies often looks markedly different than in rodent studies – antibodies don’t recognize human antigens the same as in rodents, human brains may not be preserved as well as in controlled animal studies, and human brain tissue is obtained when an unhealthy person dies. Thus, it can be very difficult to interpret human data based on what is known about adult neurogenesis in rodents.
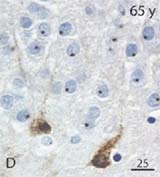
DCX-expressing cells in a 65 year-old hippocampus
However, the recent study by Knoth et al. in PLoS One does a pretty good job of getting around at least some of these problems and is perhaps the most informative study of adult neurogenesis in humans since the original Eriksson study. The authors focus their study on doublecortin (DCX), a protein involved in cell migration and the extension of neuronal processes. DCX can be expressed in mature neurons but, within the dentate gyrus, it is a reliable and specific marker of immature neurons…at least in rodents. Whether or not DCX can be applied to human studies of adult neurogenesis is a legitimate concern. Previous studies have found putative DCX-expressing cells in human tissue but these cells often lacked dendrites and axons and so it was questionable whether these cells were in fact neuronal (or sometimes even cellular).
To get at these issues, Knoth et al. examined a large number of subjects spanning a huge age range (0 to 100 years!). Since neurogenesis declines with age in rodents and primates, if DCX is truly labeling immature neurons, and if neurogenesis in humans parallels that in rodents, you’d expect the numbers of DCX+ cells to decrease with age. And this is exactly what Knoth et al. report: the density of DCX+ cells decreases roughly tenfold from puberty to old age (their Fig. 9), a drop that is pretty similar to what is seen in rodents. It is also nice to see, in their Fig. 4, some pictures of DCX+ cells that actually resemble neurons. Some cells look like those I’ve seen before (e.g. their Fig. 4e,f) but the cells in a 65 year-old subject and those in the 9 day-old subject have dendritic processes and give the reader confidence that this DCX staining is real. Interestingly, Boekhoorn et al. have shown that, as the interval between death and brain fixation increases (which is quite variable in preservation of human tissue), DCX-expressing cells lose their dendritic arbor but do not altogether disappear. Therefore, it is likely that any potential morphologic variability caused by delayed fixation did not prevent Knoth et al. from accurately quantifying DCX+ immature neurons. It is also worth noting that their estimate of the magnitude of neurogenesis, ~1-10 DCX+ cells/mm² in adulthood is in the same ballpark as reported by Eriksson (humans) and me (rats) for BrdU-labeled cells during middle to old age (~100s of cells/mm³).
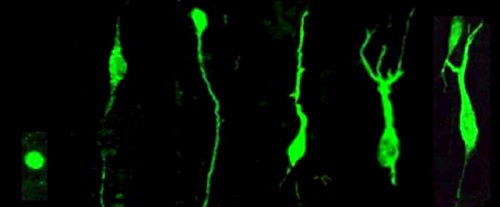
Using DCX as a marker of young neurons, Knoth et al. then go on to co-label DCX with other markers of proliferating cells and immature neurons, to better characterize the types of cells present in the adult human dentate gyrus, and to further validate DCX as a true marker of immature cells in humans. They found DCX+ cells also expressed various maturational markers previously characterized in rodents (e.g. proliferation: Ki67, PCNA; neuronal lineage: NeuN, calretinin; and many other markers I won’t pretend to understand). It would be nice to see the proportion of DCX+ cells that express the various markers, to see if the DCX population resembles that of rodents (e.g. is the proliferating proportion of a similar size? What about the “mature” proportion that co-expresses NeuN?). Regardless, the number of markers examined is very extensive and is certainly consistent with the idea that these DCX+ cells are adult-generated and go through distinct phases of maturation.
Morphology of DCX+ cells observed by Knoth et al., ranging from immature (left) to mature (right)
Likely due to the limitations of working with human tissue, many findings are qualitative and will have to be definitively answered in the future. For example, the oldest age at which DCX+ cells were still found to be proliferating depended on which endogenous marker of proliferation was used (Ki67 – 38yr, Mcm2 – 65yr, PCNA – oldest age). Depending on the validity of these markers, it is possible that proliferation ends in middle age and that DCX+ cells found in the oldest subjects are the result of a very protracted cellular maturation process. (Species differences in maturation have been found and there are hints that the neurogenesis process may be slower in primates than in rodents – e.g. cell addition and death are delayed, maturation markers may be delayed). Or does neuronal proliferation occur in old age but is simply too low to reliably detect without sufficient sampling? I also I would love to see low magnification pictures to get a better sense of how immunostaining for the various neurogenesis markers in humans compares to that of rodents, but I’m guessing that’s easier said than done.
All in all, this paper is a welcome addition to neurogenesis literature. And any wishes for more pictures or experiments has to be qualified with the disclosure that, until BrdU in injected into humans, I will always be begging for more. While on this topic – is there any way to get another human study that uses BrdU (or similar) to birthdate new neurons, and provide concrete evidence of the magnitude and maturational profile of adult neurogenesis? Once upon a time BrdU was used extensively to both treat and evaluate cancer. Might there be some BrdU-containing tissue hidden away in lab storage somewhere? Alternatively, many great discoveries have been made, and Nobels awarded, in the name of self-experimentation. Is there any good reason to suspect that a single glass of BrdU water is going to harm any of us? Probably could get a great paper out of it. Then again, we might not be alive to read it…or might not have a hippocampus with which to remember reading it, but we’re not doing science for ourselves anyway, are we?
Knoth, R., Singec, I., Ditter, M., Pantazis, G., Capetian, P., Meyer, R., Horvat, V., Volk, B., & Kempermann, G. (2010). Murine Features of Neurogenesis in the Human Hippocampus across the Lifespan from 0 to 100 Years PLoS ONE, 5 (1) DOI: 10.1371/journal.pone.0008809


No comments:
Post a Comment