This appears to be the first good research showing why SSRIs do not work for most people in treating depression and anxiety. It appears that early life stress increases serotonin levels in the brain to the point that a negative feedback loop develops, reducing the brain's sensitivity to the serotonin. The resulting depression and/or anxiety that develops is exacerbated by SSRIs, which just add to the existing surplus of serotonin in the raphe nucleus and reduces serotonin in hippocampus, where it is needed most.
Early life stress may cause excess serotonin release resulting in a serotonin deficit
Data suggest a reason why SSRI medications may fail in many patientsHere are the abstracts from the original articles, both of which are open access.
Studies indicate that the majority of people with mood and anxiety disorders who receive the most commonly prescribed class of antidepressant medications, Selective Serotonin Reuptake Inhibitors or SSRI's, are not helped by these medications. SSRIs are designed to increase serotonin, a neurotransmitter in the brain that is key to maintenance of mood.
Researchers led by Jeremy D. Coplan, MD, professor of psychiatry at SUNY Downstate Medical Center, have published data suggesting an explanation for the longstanding puzzle as to why low serotonin could not be detected in depression without suicidal intent, even though many antidepressant treatments work by increasing serotonin in areas key for mood regulation, such as the hippocampus. The pre-clinical research was published in a recent edition of Frontiers in Behavioral Neuroscience.
Dr. Coplan explains, "We have shown that serotonin is too high near the serotonin brain cells, reducing firing of the serotonin nerve cells through a well-documented negative feedback mechanism in the raphe nucleus. The result is that the hippocampus and other critical brain structures needed for mood maintenance do not get enough serotonin. We can see this because the hippocampus is shrunken and the white matter loses integrity. By the time serotonin metabolites are measured in a lumbar spinal tap, the usual way serotonin levels have been measured, the high serotonin has mixed with the low serotonin and you have no difference from people who are healthy."
He continues, "We have hypothesized in an earlier paper that this is a plausible reason why SSRIs may not work in a majority of people, because SSRIs will tend to make the high serotonin even higher in the raphe nucleus. The serotonin neuron may not be able to adapt and restore its firing, inducing a presumed serotonin deficit in terminal fields, evidenced by shrinkage of the hippocampus."
He adds, "We cannot say categorically, in our pre-clinical model, that high serotonin in the raphe nucleus leads to low serotonin in the hippocampus, but studies by J. John Mann, MD, a co-author on the paper, and Victoria Arango, PhD, both of Columbia University Medical Center, have shown that people who committed suicide exhibited high serotonin in the raphe nucleus and low serotonin in another area of the brain critical for mood maintenance, the prefrontal cortex. Additional studies should be performed, especially since better understanding of the serotonin system will significantly improve future treatment options."
In the earlier paper, also in Frontiers in Behavioral Neuroscience, Dr. Coplan proposed augmentation therapies in treatment-resistant patients, including stacking one medication upon another in the most difficult cases: "This is what physicians do for hypertension, diabetes, and congestive heart failure," said Dr. Coplan. "But in psychiatry, we sometimes act as if our medications are so effective that we are exempt from how the rest of medicine deals with difficult-to-treat cases."
Other approaches to bypass the high midbrain serotonin impasse, according to Dr. Coplan, are shutting glutamate input into the raphe nucleus, a portion of the brain that controls the release of serotonin, and utilizing drugs that block noradrenergic input into the dorsal raphe.
Dr. Coplan notes that a recent large-scale study showed only a minority of patients do well on SSRIs, and of those, many lose response in a year or two. "There is an epidemic of inadequately treated depression and psychiatrists are not well trained to deal with this challenge," he observed. "What they often do is change from one antidepressant to another when there is a lack of response. Eventually the patient becomes non-compliant and the patient, rather than the treatment, is blamed for the non-efficacy."
"These two papers provide possible insights as to why our treatments are ineffective and what we should be doing to treat patients effectively," Dr. Coplan said. "Many academic researchers currently do not practice clinically, so they are out of touch with real-life patients and their struggles. In the meantime, suicide rates have not budged in decades."
Elevated cerebrospinal fluid 5-hydroxyindoleacetic acid in macaques following early life stress and inverse association with hippocampal volume: preliminary implications for serotonin-related function in mood and anxiety disorders
- 1Nonhuman Primate Laboratory, Department of Psychiatry and Behavioral Sciences, Downstate Medical Center, State University of New York, Brooklyn, NY, USA
- 2Geriatric Psychiatry, New York State Psychiatric Institute, New York, NY, USA
- 3College of Medicine, State University of New York Downstate Medical Center, Brooklyn, NY, USA
- 4Departamento de Psiquiatria & Neuroradiologia, Universidade Federal de São Paulo, São Paulo, Brazil
- 5Franklin Behavioral Health Consultants, Bronx, NY, USA
- 6Department of Molecular Imaging and Neuropathology, New York State Psychiatric Institute, New York, NY, USA
- 7Departments of Psychiatry, Neuroscience, and Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, USA
- 8Fishberg Department of Neuroscience and Friedman Brain Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA
- 9Department of Psychiatry, Yale University School of Medicine, New Haven, CT, USA
- 10Mental Health Care Line, Michael E. Debakey VA Medical Center, Houston, TX, USA
- 11Menninger Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX, USA
- 12Child Study Center, Yale University School of Medicine, New Haven, CT, USA
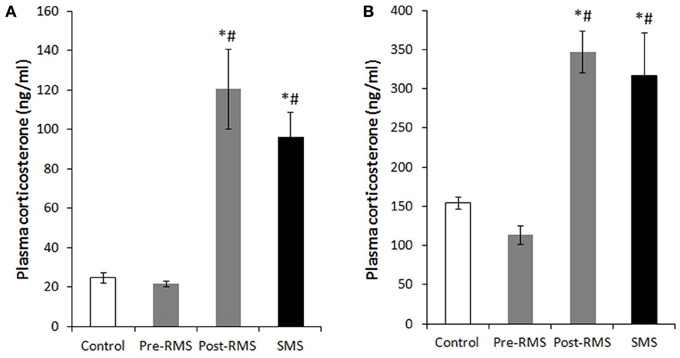
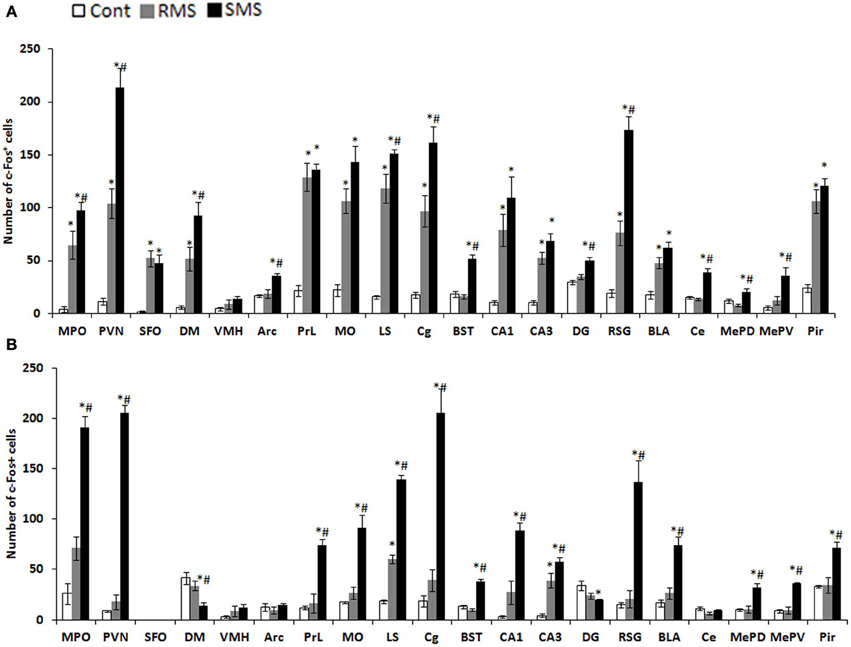
Background: Early life stress (ELS) is cited as a risk for mood and anxiety disorders, potentially through altered serotonin neurotransmission. We examined the effects of ELS, utilizing the variable foraging demand (VFD) macaque model, on adolescent monoamine metabolites. We sought to replicate an increase in cerebrospinal fluid (CSF) 5-hydroxyindoleacetic acid (5-HIAA) observed in two previous VFD cohorts. We hypothesized that elevated cisternal 5-HIAA was associated with reduced neurotrophic effects, conceivably due to excessive negative feedback at somatodendritic 5-HT1A autoreceptors. A putatively decreased serotonin neurotransmission would be reflected by reductions in hippocampal volume and white matter (WM) fractional anisotropy (FA).
Methods: When infants were 2–6 months of age, bonnet macaque mothers were exposed to VFD. We employed cisternal CSF taps to measure monoamine metabolites in VFD (N = 22) and non-VFD (N = 14) offspring (mean age = 2.61 years). Metabolites were correlated with hippocampal volume obtained by MRI and WM FA by diffusion tensor imaging in young adulthood in 17 males [10 VFD (mean age = 4.57 years)].
Results: VFD subjects exhibited increased CSF 5-HIAA compared to non-VFD controls. An inverse correlation between right hippocampal volume and 5-HIAA was noted in VFD- but not controls. CSF HVA and MHPG correlated inversely with hippocampal volume only in VFD. CSF 5-HIAA correlated inversely with FA of the WM tracts of the anterior limb of the internal capsule (ALIC) only in VFD.
Conclusions: Elevated cisternal 5-HIAA in VFD may reflect increased dorsal raphe serotonin, potentially inducing excessive autoreceptor activation, inducing a putative serotonin deficit in terminal fields. Resultant reductions in neurotrophic activity are reflected by smaller right hippocampal volume. Convergent evidence of reduced neurotrophic activity in association with high CSF 5-HIAA in VFD was reflected by reduced FA of the ALIC.Full Citation:Coplan JD, Fulton SL, Reiner W, Jackowski A, Panthangi V, Perera TD, Gorman JM, Huang Y, Tang CY, Hof PR, Kaffman A, Dwork AJ, Mathew SJ, Kaufman J and Mann JJ (2014, Dec 24). Elevated cerebrospinal fluid 5-hydroxyindoleacetic acid in macaques following early life stress and inverse association with hippocampal volume: preliminary implications for serotonin-related function in mood and anxiety disorders. Front. Behav. Neurosci. 8:440. doi: 10.3389/fnbeh.2014.00440
* * * * *
A neurobiological hypothesis of treatment-resistant depression – mechanisms for selective serotonin reuptake inhibitor non-efficacy
First-line treatment of major depression includes administration of a selective serotonin reuptake inhibitor (SSRI), yet studies suggest that remission rates following two trials of an SSRI are <50%. The authors examine the putative biological substrates underlying “treatment resistant depression (TRD)” with the goal of elucidating novel rationales to treat TRD. We look at relevant articles from the preclinical and clinical literature combined with clinical exposure to TRD patients. A major focus was to outline pathophysiological mechanisms whereby the serotonin system becomes impervious to the desired enhancement of serotonin neurotransmission by SSRIs. A complementary focus was to dissect neurotransmitter systems, which serve to inhibit the dorsal raphe. We propose, based on a body of translational studies, TRD may not represent a simple serotonin deficit state but rather an excess of midbrain peri-raphe serotonin and subsequent deficit at key fronto-limbic projection sites, with ultimate compromise in serotonin-mediated neuroplasticity. Glutamate, serotonin, noradrenaline, and histamine are activated by stress and exert an inhibitory effect on serotonin outflow, in part by “flooding” 5-HT1A autoreceptors by serotonin itself. Certain factors putatively exacerbate this scenario – presence of the short arm of the serotonin transporter gene, early-life adversity and comorbid bipolar disorder – each of which has been associated with SSRI-treatment resistance. By utilizing an incremental approach, we provide a system for treating the TRD patient based on a strategy of rescuing serotonin neurotransmission from a state of SSRI-induced dorsal raphe stasis. This calls for “stacked” interventions, with an SSRI base, targeting, if necessary, the glutamatergic, serotonergic, noradrenergic, and histaminergic systems, thereby successively eliminating the inhibitory effects each are capable of exerting on serotonin neurons. Future studies are recommended to test this biologically based approach for treatment of TRD.
- 1Division of Neuropsychopharmacology, Department of Psychiatry and Behavioral Science, State University of New York Downstate Medical Center, Brooklyn, NY, USA
- 2Department of Psychiatry, Yale School of Medicine, New Haven, CT, USA
- 3Clinical Neuroscience Division, National Center for PTSD, West Haven, CT, USA
- 4State University of New York Downstate College of Medicine, Brooklyn, NY, USA
Full Citation:Coplan JD, Gopinath S, Abdallah CG and Berry BR. (2014, May 20). A neurobiological hypothesis of treatment-resistant depression – mechanisms for selective serotonin reuptake inhibitor non-efficacy. Front. Behav. Neurosci. 8:189. doi: 10.3389/fnbeh.2014.00189