Turmeric, and its active ingredient, curcumin, is one of nature's wonderful gifts. Not only is it the foundation for most curries (yum!), but it is also turning out to have impressive and far reaching health benefits, including:
- Acts as an anti-inflammatory pain killer
- Triggers apoptosis in cancer cells
- Inhibits the amyloid plaques that cause Alzheimer's Disease
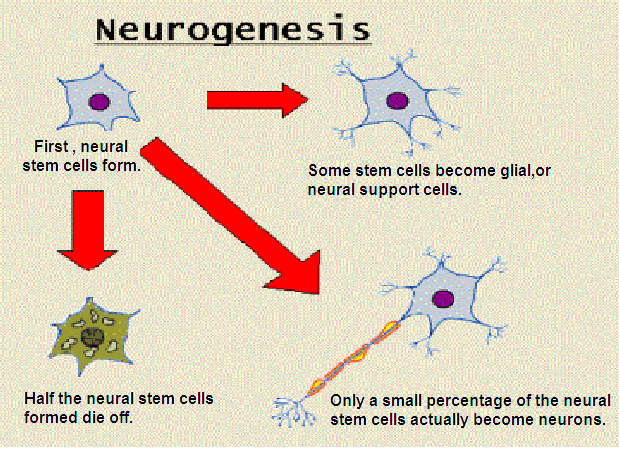
- Enables the brain to generate new stem cells for neurons
Let's start with a summary of the research from Science Daily:
Turmeric compound boosts regeneration of brain stem cells
Date: September 25, 2014
Source: BioMed Central
Summary:A bioactive compound found in turmeric promotes stem cell proliferation and differentiation in the brain, reveals new research published today in the open access journal Stem Cell Research & Therapy. The findings suggest aromatic turmerone could be a future drug candidate for treating neurological disorders, such as stroke and Alzheimer's disease.
A bioactive compound found in turmeric promotes stem cell proliferation and differentiation in the brain, reveals new research. The findings suggest aromatic turmerone could be a future drug candidate for treating neurological disorders, such as stroke and Alzheimer's disease.
The study looked at the effects of aromatic (ar-) turmerone on endogenous neutral stem cells (NSC), which are stem cells found within adult brains. NSC differentiate into neurons, and play an important role in self-repair and recovery of brain function in neurodegenerative diseases. Previous studies of ar-turmerone have shown that the compound can block activation of microglia cells. When activated, these cells cause neuroinflammation, which is associated with different neurological disorders. However, ar-turmerone's impact on the brain's capacity to self-repair was unknown.
Researchers from the Institute of Neuroscience and Medicine in Jülich, Germany, studied the effects of ar-turmerone on NSC proliferation and differentiation both in vitro and in vivo. Rat fetal NSC were cultured and grown in six different concentrations of ar-turmerone over a 72 hour period. At certain concentrations, ar-turmerone was shown to increase NSC proliferation by up to 80%, without having any impact on cell death. The cell differentiation process also accelerated in ar-turmerone-treated cells compared to untreated control cells.
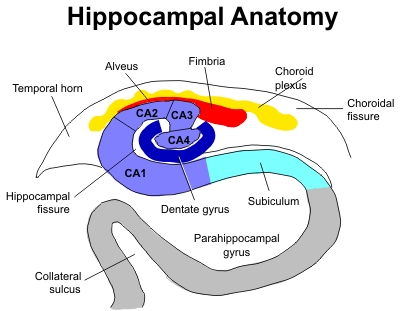
To test the effects of ar-turmerone on NSC in vivo, the researchers injected adult rats with ar-turmerone. Using PET imaging and a tracer to detect proliferating cells, they found that the subventricular zone (SVZ) was wider, and the hippocampus expanded, in the brains of rats injected with ar-turmerone than in control animals. The SVZ and hippocampus are the two sites in adult mammalian brains where neurogenesis, the growth of neurons, is known to occur.
Lead author of the study, Adele Rueger, said: "While several substances have been described to promote stem cell proliferation in the brain, fewer drugs additionally promote the differentiation of stem cells into neurons, which constitutes a major goal in regenerative medicine. Our findings on aromatic turmerone take us one step closer to achieving this goal."
Ar-turmerone is the lesser-studied of two major bioactive compounds found in turmeric. The other compound is curcumin, which is well known for its anti-inflammatory and neuroprotective properties.
Story Source:
The above story is based on materials provided by BioMed Central. Note: Materials may be edited for content and length.
Journal Reference:
Joerg Hucklenbroich, Rebecca Klein, Bernd Neumaier, Rudolf Graf, Gereon Fink, Michael Schroeter, Maria Rueger. (2014, Sep 26). Aromatic-turmerone induces neural stem cell proliferation in vitro and in vivo. Stem Cell Research & Therapy; 5(4): 100 DOI: 10.1186/scrt500
* * * * *
Here is the beginning of the article, including the abstract and the introduction. The article is open access.
Aromatic-turmerone induces neural stem cell proliferation in vitro and in vivo
Joerg Hucklenbroich [1,2], Rebecca Klein [2,3], Bernd Neumaier [3], Rudolf Graf [3], Gereon Rudolf Fink [1,2], Michael Schroeter [1,2,3] and Maria Adele Rueger [1,2,3]
1, Cognitive Neuroscience, Institute of Neuroscience and Medicine (INM-3), Research Centre Juelich, Leo-Brandt-Straße 52425, Jülich, GermanyThis is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
2. Department of Neurology, University Hospital of Cologne, Cologne, Germany
3. Max Planck Institute for Neurological Research, Cologne, Germany
Stem Cell Research & Therapy 2014, 5(4):100 doi:10.1186/scrt500
Abstract
Introduction
Aromatic (ar-) turmerone is a major bioactive compound of the herb Curcuma longa. It has been suggested that ar-turmerone inhibits microglia activation, a property that may be useful in treating neurodegenerative disease. Furthermore, the effects of ar-turmerone on neural stem cells (NSCs) remain to be investigated.
Methods
We exposed primary fetal rat NSCs to various concentrations of ar-turmerone. Thereafter, cell proliferation and differentiation potential were assessed. In vivo, naïve rats were treated with a single intracerebroventricular (i.c.v.) injection of ar-turmerone. Proliferative activity of endogenous NSCs was assessed in vivo, by using noninvasive positron emission tomography (PET) imaging and the tracer [18F]-fluoro-L-thymidine ([18F]FLT), as well as ex vivo.
Results
In vitro, ar-turmerone increased dose-dependently the number of cultured NSCs, because of an increase in NSC proliferation (P < 0.01). Proliferation data were supported by qPCR-data for Ki-67 mRNA. In vitro as well as in vivo, ar-turmerone promoted neuronal differentiation of NSCs. In vivo, after i.c.v. injection of ar-turmerone, proliferating NSCs were mobilized from the subventricular zone (SVZ) and the hippocampus of adult rats, as demonstrated by both [18F]FLT-PET and histology (P < 0.05).
Conclusions
Both in vitro and in vivo data suggest that ar-turmerone induces NSC proliferation. Ar-turmerone thus constitutes a promising candidate to support regeneration in neurologic disease.
Introduction
Curcumin and ar-turmerone are the major bioactive compounds of the herb Curcuma longa. Although many studies have demonstrated curcumin to possess antiinflammatory and neuroprotective properties (reviewed by [1]), to date, the effects of ar-turmerone remain to be elucidated. For example, antitumor properties, exerted via the induction of apoptosis [2] and inhibition of tumor cell invasion [3], have been attributed to ar-turmerone. Park et al. [4,5] recently suggested that ar-turmerone also possesses antiinflammatory properties resulting from the blockade of key signaling pathways in microglia. Because microglia activation is a hallmark of neuroinflammation and is associated with various neurologic disorders, including neurodegenerative diseases [6,7] and stroke [8,9], ar-turmerone constitutes a promising therapeutic agent for various neurologic disorders.
The regenerative potential of endogenous neural stem cells (NSCs) plays an important role in neurodegenerative disease and stroke. Endogenous NSCs are mobilized by cerebral ischemia [10] as well as by various neurodegenerative diseases [11,12], although their intrinsic regenerative response is insufficient to enable functional recovery. The targeted (that is, pharmacologic) activation of endogenous NSCs has been shown to enhance self-repair and recovery of function in the adult brain in both stroke [13,14] and neurodegeneration [15]. Importantly, NSCs and microglia relevantly interact with each other, thereby affecting their respective functions [16,17].
Thus, with the perspective of ar-turmerone as a therapeutic option in mind, we investigated the effects of ar-turmerone on NSCs in vitro and in vivo.
* * * * *
Here is a collection of articles complied by Examiner.com on their page on curcumin. Some of these studies show that curcumin is not effective for certain conditions. For a short-cut to seeing where curcumin is effective, the Examiner page has a handy little chart - The Human Effect Matrix.
- Chemistry and biological activities of C. longa
- Kiuchi F, et al. Nematocidal activity of turmeric: synergistic action of curcuminoids. Chem Pharm Bull (Tokyo). (1993)
- Changtam C, et al. Curcuminoid analogs with potent activity against Trypanosoma and Leishmania species. Eur J Med Chem. (2010)
- Qualitative and quantitative analysis of curcuminoids in herbal medicines derived from Curcuma species
- Chang CJ, et al. Beneficial impact of Zingiber zerumbet on insulin sensitivity in fructose-fed rats. Planta Med. (2012)
- Zhou H, Beevers CS, Huang S. The targets of curcumin. Curr Drug Targets. (2011)
- Usharani P, et al. Effect of NCB-02, atorvastatin and placebo on endothelial function, oxidative stress and inflammatory markers in patients with type 2 diabetes mellitus: a randomized, parallel-group, placebo-controlled, 8-week study. Drugs R D. (2008)
- Venkataranganna MV, et al. NCB-02 (standardized Curcumin preparation) protects dinitrochlorobenzene- induced colitis through down-regulation of NFkappa-B and iNOS. World J Gastroenterol. (2007)
- Esatbeyoglu T, et al. Curcumin-from molecule to biological function. Angew Chem Int Ed Engl. (2012)
- Scotter MJ. Methods for the determination of European Union-permitted added natural colours in foods: a review. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. (2011)
- Wang YJ, et al. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal. (1997)
- Sharma RA, et al. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res. (2001)
- Lao CD, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. (2006)
- Cheng AL, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. (2001)
- Dhillon N, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. (2008)
- Shoba G, et al. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. (1998)
- Marczylo TH, et al. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol. (2007)
- Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. (2009)
- Cuomo J, et al. Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation. J Nat Prod. (2011)
- Sasaki H, et al. Innovative preparation of curcumin for improved oral bioavailability. Biol Pharm Bull. (2011)
- Zhongfa L, et al. Enhancement of curcumin oral absorption and pharmacokinetics of curcuminoids and curcumin metabolites in mice. Cancer Chemother Pharmacol. (2012)
- Kanai M, et al. Dose-escalation and pharmacokinetic study of nanoparticle curcumin, a potential anticancer agent with improved bioavailability, in healthy human volunteers. Cancer Chemother Pharmacol. (2012)
- Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. (1997)
- Angel P, et al. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. (1987)
- Dhandapani KM, Mahesh VB, Brann DW. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP-1 and NFkappaB transcription factors. J Neurochem. (2007)
- Bierhaus A, et al. The dietary pigment curcumin reduces endothelial tissue factor gene expression by inhibiting binding of AP-1 to the DNA and activation of NF-kappa B. Thromb Haemost. (1997)
- Dickinson DA, et al. Curcumin alters EpRE and AP-1 binding complexes and elevates glutamate-cysteine ligase gene expression. FASEB J. (2003)
- Beevers CS, et al. Curcumin disrupts the Mammalian target of rapamycin-raptor complex. Cancer Res. (2009)
- Yu S, et al. Curcumin inhibits Akt/mammalian target of rapamycin signaling through protein phosphatase-dependent mechanism. Mol Cancer Ther. (2008)
- Takeuchi T, et al. Structural relationship of curcumin derivatives binding to the BRCT domain of human DNA polymerase lambda. Genes Cells. (2006)
- Leu TH, et al. Direct inhibitory effect of curcumin on Src and focal adhesion kinase activity. Biochem Pharmacol. (2003)
- Reddy S, Aggarwal BB. Curcumin is a non-competitive and selective inhibitor of phosphorylase kinase. FEBS Lett. (1994)
- Lee CW, et al. Transcriptional regulation of VCAM-1 expression by tumor necrosis factor-alpha in human tracheal smooth muscle cells: involvement of MAPKs, NF-kappaB, p300, and histone acetylation. J Cell Physiol. (2006)
- Fang J, Lu J, Holmgren A. Thioredoxin reductase is irreversibly modified by curcumin: a novel molecular mechanism for its anticancer activity. J Biol Chem. (2005)
- Skrzypczak-Jankun E, et al. Structure of curcumin in complex with lipoxygenase and its significance in cancer. Int J Mol Med. (2003)
- Gupta KK, et al. Dietary antioxidant curcumin inhibits microtubule assembly through tubulin binding. FEBS J. (2006)
- Hu GX, et al. Curcumin derivatives inhibit testicular 17beta-hydroxysteroid dehydrogenase 3. Bioorg Med Chem Lett. (2010)
- Liao S, et al. Growth suppression of hamster flank organs by topical application of catechins, alizarin, curcumin, and myristoleic acid. Arch Dermatol Res. (2001)
- Bustanji Y, et al. Inhibition of glycogen synthase kinase by curcumin: Investigation by simulated molecular docking and subsequent in vitro/in vivo evaluation. J Enzyme Inhib Med Chem. (2009)
- Shin HK, et al. Inhibitory effect of curcumin on motility of human oral squamous carcinoma YD-10B cells via suppression of ERK and NF-kappaB activations. Phytother Res. (2010)
- Wang Z, et al. Notch-1 down-regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells. Cancer. (2006)
- Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. (1999)
- Aggarwal S, et al. Inhibition of growth and survival of human head and neck squamous cell carcinoma cells by curcumin via modulation of nuclear factor-kappaB signaling. Int J Cancer. (2004)
- Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) {corrected}. J Biol Chem. (1995)
- Sung B, et al. Cancer cell signaling pathways targeted by spice-derived nutraceuticals. Nutr Cancer. (2012)
- Anand P1, et al. Bioavailability of curcumin: problems and promises. Mol Pharm. (2007)
- Comparative absorption of curcumin formulations
- Yallapu MM1, Jaggi M, Chauhan SC. Curcumin nanoformulations: a future nanomedicine for cancer. Drug Discov Today. (2012)
- Liu A1, et al. Validated LC/MS/MS assay for curcumin and tetrahydrocurcumin in rat plasma and application to pharmacokinetic study of phospholipid complex of curcumin. J Pharm Biomed Anal. (2006)
- Yu H1, Huang Q. Improving the oral bioavailability of curcumin using novel organogel-based nanoemulsions. J Agric Food Chem. (2012)
- Hu L1, et al. Preparation and enhancement of oral bioavailability of curcumin using microemulsions vehicle. J Agric Food Chem. (2012)
- Khalil NM1, et al. Pharmacokinetics of curcumin-loaded PLGA and PLGA-PEG blend nanoparticles after oral administration in rats. Colloids Surf B Biointerfaces. (2013)
- Antony B1, et al. A Pilot Cross-Over Study to Evaluate Human Oral Bioavailability of BCM-95CG (Biocurcumax), A Novel Bioenhanced Preparation of Curcumin. Indian J Pharm Sci. (2008)
- Kulkarni SK1, Akula KK, Deshpande J. Evaluation of antidepressant-like activity of novel water-soluble curcumin formulations and St. John's wort in behavioral paradigms of despair. Pharmacology. (2012)
- Garcea G, et al. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers Prev. (2005)
- Mandeville JS, Froehlich E, Tajmir-Riahi HA. Study of curcumin and genistein interactions with human serum albumin. J Pharm Biomed Anal. (2009)
- Ireson C, et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. (2001)
- Vareed SK, et al. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev. (2008)
- Holder GM, Plummer JL, Ryan AJ. The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the rat. Xenobiotica. (1978)
- Wang R, et al. Curcumin protects against glutamate excitotoxicity in rat cerebral cortical neurons by increasing brain-derived neurotrophic factor level and activating TrkB. Brain Res. (2008)
- Matteucci A, et al. Curcumin protects against NMDA-induced toxicity: a possible role for NR2A subunit. Invest Ophthalmol Vis Sci. (2011)
- Chen RW, et al. Regulation of c-Jun N-terminal kinase, p38 kinase and AP-1 DNA binding in cultured brain neurons: roles in glutamate excitotoxicity and lithium neuroprotection. J Neurochem. (2003)
- Matteucci A, et al. Curcumin treatment protects rat retinal neurons against excitotoxicity: effect on N-methyl-D: -aspartate-induced intracellular Ca(2+) increase. Exp Brain Res. (2005)
- Wu A, Ying Z, Gomez-Pinilla F. Dietary curcumin counteracts the outcome of traumatic brain injury on oxidative stress, synaptic plasticity, and cognition. Exp Neurol. (2006)
- Nair SM, et al. Corticosteroid regulation of ion channel conductances and mRNA levels in individual hippocampal CA1 neurons. J Neurosci. (1998)
- Xu Y, et al. Curcumin reverses impaired cognition and neuronal plasticity induced by chronic stress. Neuropharmacology. (2009)
- Frautschy SA, et al. Phenolic anti-inflammatory antioxidant reversal of Abeta-induced cognitive deficits and neuropathology. Neurobiol Aging. (2001)
- Sharma S, et al. Dietary curcumin supplementation counteracts reduction in levels of molecules involved in energy homeostasis after brain trauma. Neuroscience. (2009)
- Yang F, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. (2005)
- Baum L, et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol. (2008)
- Reinke AA, Gestwicki JE. Structure-activity relationships of amyloid beta-aggregation inhibitors based on curcumin: influence of linker length and flexibility. Chem Biol Drug Des. (2007)
- Ma QL, et al. Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: suppression by omega-3 fatty acids and curcumin. J Neurosci. (2009)
- Curcumin Inhibits Formation of Amyloid β Oligomers and Fibrils, Binds Plaques, and Reduces Amyloid in Vivo
- A Diet Enriched with the Omega-3 Fatty Acid Docosahexaenoic Acid Reduces Amyloid Burden in an Aged Alzheimer Mouse Model
- Gomez-Pinilla F. Collaborative effects of diet and exercise on cognitive enhancement. Nutr Health. (2011)
- Wu A, Ying Z, Gomez-Pinilla F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience. (2008)
- Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as "Curecumin": from kitchen to clinic. Biochem Pharmacol. (2008)
- Di Pierro F, et al. Comparative evaluation of the pain-relieving properties of a lecithinized formulation of curcumin (Meriva(®)), nimesulide, and acetaminophen. J Pain Res. (2013)
- Belcaro G, et al. Product-evaluation registry of Meriva®, a curcumin-phosphatidylcholine complex, for the complementary management of osteoarthritis. Panminerva Med. (2010)
- Belcaro G, et al. Efficacy and safety of Meriva®, a curcumin-phosphatidylcholine complex, during extended administration in osteoarthritis patients. Altern Med Rev. (2010)
- Agarwal KA, et al. Efficacy of turmeric (curcumin) in pain and postoperative fatigue after laparoscopic cholecystectomy: a double-blind, randomized placebo-controlled study. Surg Endosc. (2011)
- Morimoto T, et al. The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats. J Clin Invest. (2008)
- Storka A, et al. Effect of liposomal curcumin on red blood cells in vitro. Anticancer Res. (2013)
- Tang W, et al. Association of sICAM-1 and MCP-1 with coronary artery calcification in families enriched for coronary heart disease or hypertension: the NHLBI Family Heart Study. BMC Cardiovasc Disord. (2007)
- DiSilvestro RA, et al. Diverse effects of a low dose supplement of lipidated curcumin in healthy middle aged people. Nutr J. (2012)
- Fang XD, et al. Curcumin ameliorates high glucose-induced acute vascular endothelial dysfunction in rat thoracic aorta. Clin Exp Pharmacol Physiol. (2009)
- Majithiya JB, Balaraman R. Time-dependent changes in antioxidant enzymes and vascular reactivity of aorta in streptozotocin-induced diabetic rats treated with curcumin. J Cardiovasc Pharmacol. (2005)
- Sompamit K, et al. Curcumin improves vascular function and alleviates oxidative stress in non-lethal lipopolysaccharide-induced endotoxaemia in mice. Eur J Pharmacol. (2009)
- El-Bassossy HM, et al. Haem oxygenase-1 induction protects against tumour necrosis factor alpha impairment of endothelial-dependent relaxation in rat isolated pulmonary artery. Br J Pharmacol. (2009)
- Curcumin binds tubulin, induces mitotic catastrophe, and impedes normal endothelial cell proliferation
- Akazawa N, et al. Curcumin ingestion and exercise training improve vascular endothelial function in postmenopausal women. Nutr Res. (2012)
- Fleenor BS, et al. Curcumin ameliorates arterial dysfunction and oxidative stress with aging. Exp Gerontol. (2013)
- Nakmareong S, et al. Antioxidant and vascular protective effects of curcumin and tetrahydrocurcumin in rats with L-NAME-induced hypertension. Naunyn Schmiedebergs Arch Pharmacol. (2011)
- Sagiroglu T, et al. Protective effect of curcumin on cyclosporin A-induced endothelial dysfunction, antioxidant capacity, and oxidative damage. Toxicol Ind Health. (2012)
- Khajehdehi P, et al. Oral supplementation of turmeric decreases proteinuria, hematuria, and systolic blood pressure in patients suffering from relapsing or refractory lupus nephritis: a randomized and placebo-controlled study. J Ren Nutr. (2012)
- Pungcharoenkul K, Thongnopnua P. Effect of different curcuminoid supplement dosages on total in vivo antioxidant capacity and cholesterol levels of healthy human subjects. Phytother Res. (2011)
- Kim T, et al. Curcumin activates AMPK and suppresses gluconeogenic gene expression in hepatoma cells. Biochem Biophys Res Commun. (2009)
- AMP-activated protein kinase: the energy charge hypothesis revisited
- Ikonomov OC, et al. Requirement for PIKfyve enzymatic activity in acute and long-term insulin cellular effects. Endocrinology. (2002)
- Yang X, et al. Curcumin inhibits platelet-derived growth factor-stimulated vascular smooth muscle cell function and injury-induced neointima formation. Arterioscler Thromb Vasc Biol. (2006)
- Srinivasan M. Effect of curcumin on blood sugar as seen in a diabetic subject. Indian J Med Sci. (1972)
- Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. (2009)
- Kim JH, et al. Curcumin stimulates glucose uptake through AMPK-p38 MAPK pathways in L6 myotube cells. J Cell Physiol. (2010)
- Kang C, Kim E. Synergistic effect of curcumin and insulin on muscle cell glucose metabolism. Food Chem Toxicol. (2010)
- Pan W, et al. AMPK mediates curcumin-induced cell death in CaOV3 ovarian cancer cells. Oncol Rep. (2008)
- Weisberg SP, Leibel R, Tortoriello DV. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology. (2008)
- Yekollu SK, Thomas R, O'Sullivan B. Targeting curcusomes to inflammatory dendritic cells inhibits NF-κB and improves insulin resistance in obese mice. Diabetes. (2011)
- Chuengsamarn S, et al. Curcumin extract for prevention of type 2 diabetes. Diabetes Care. (2012)
- Xie XY, et al. Curcumin attenuates lipolysis stimulated by tumor necrosis factor-α or isoproterenol in 3T3-L1 adipocytes. Phytomedicine. (2012)
- Soltoff SP, Hedden L. Regulation of ERK1/2 by ouabain and Na-K-ATPase-dependent energy utilization and AMPK activation in parotid acinar cells. Am J Physiol Cell Physiol. (2008)
- Lee YK, et al. Curcumin exerts antidifferentiation effect through AMPKalpha-PPAR-gamma in 3T3-L1 adipocytes and antiproliferatory effect through AMPKalpha-COX-2 in cancer cells. J Agric Food Chem. (2009)
- Zhao J, et al. Suppression of fatty acid synthase, differentiation and lipid accumulation in adipocytes by curcumin. Mol Cell Biochem. (2011)
- Aggarwal BB. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annu Rev Nutr. (2010)
- The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase
- Zhang HH, et al. Tumor necrosis factor-alpha stimulates lipolysis in differentiated human adipocytes through activation of extracellular signal-related kinase and elevation of intracellular cAMP. Diabetes. (2002)
- Patton JS, et al. Interferons and tumor necrosis factors have similar catabolic effects on 3T3 L1 cells. Proc Natl Acad Sci U S A. (1986)
- Ruan H, et al. Tumor necrosis factor-alpha suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes: nuclear factor-kappaB activation by TNF-alpha is obligatory. Diabetes. (2002)
- Qiao L, Shao J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J Biol Chem. (2006)
- Subauste AR, Burant CF. Role of FoxO1 in FFA-induced oxidative stress in adipocytes. Am J Physiol Endocrinol Metab. (2007)
- Ciardi C, et al. Food additives such as sodium sulphite, sodium benzoate and curcumin inhibit leptin release in lipopolysaccharide-treated murine adipocytes in vitro. Br J Nutr. (2012)
- Pongchaidecha A, et al. Effects of curcuminoid supplement on cardiac autonomic status in high-fat-induced obese rats. Nutrition. (2009)
- Ejaz A, et al. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J Nutr. (2009)
- Arbiser JL, et al. Curcumin is an in vivo inhibitor of angiogenesis. Mol Med. (1998)
- Bank J, Song DH. Curcumin Protects Against Ischemia/Reperfusion Injury in Rat Skeletal Muscle. J Surg Res. (2011)
- Avci G, et al. Curcumin protects against ischemia/reperfusion injury in rat skeletal muscle. J Surg Res. (2012)
- Pizzo P, et al. Grp94 acts as a mediator of curcumin-induced antioxidant defence in myogenic cells. J Cell Mol Med. (2010)
- Shinkai Y, Yamamoto C, Kaji T. Lead induces the expression of endoplasmic reticulum chaperones GRP78 and GRP94 in vascular endothelial cells via the JNK-AP-1 pathway. Toxicol Sci. (2010)
- Sevgiler Y, Karaytug S, Karayakar F. Antioxidative effects of N-acetylcysteine, lipoic acid, taurine, and curcumin in the muscle of Cyprinus carpio L. exposed to cadmium. Arh Hig Rada Toksikol. (2011)
- Vazeille E, et al. Curcumin treatment prevents increased proteasome and apoptosome activities in rat skeletal muscle during reloading and improves subsequent recovery. J Nutr Biochem. (2012)
- Siddiqui RA, et al. Attenuation of proteolysis and muscle wasting by curcumin c3 complex in MAC16 colon tumour-bearing mice. Br J Nutr. (2009)
- Li YP, et al. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J. (2005)
- Deng YT, et al. Suppression of free fatty acid-induced insulin resistance by phytopolyphenols in C2C12 mouse skeletal muscle cells. J Agric Food Chem. (2012)
- Na LX, et al. Curcumin improves insulin resistance in skeletal muscle of rats. Nutr Metab Cardiovasc Dis. (2011)
- Cheng TC, et al. Activation of muscarinic M-1 cholinoceptors by curcumin to increase glucose uptake into skeletal muscle isolated from Wistar rats. Neurosci Lett. (2009)
- Xavier S, et al. β(2)-Adrenoceptor and insulin receptor expression in the skeletal muscle of streptozotocin induced diabetic rats: Antagonism by vitamin D(3) and curcumin. Eur J Pharmacol. (2012)
- He HJ, et al. Curcumin attenuates Nrf2 signaling defect, oxidative stress in muscle and glucose intolerance in high fat diet-fed mice. World J Diabetes. (2012)
- Seo KI, et al. Effect of curcumin supplementation on blood glucose, plasma insulin, and glucose homeostasis related enzyme activities in diabetic db/db mice. Mol Nutr Food Res. (2008)
- Chaudhary A, Bhandari A, Pandurangan A. Antioxidant potential and total phenolic content of methanolic bark extract of Madhuca indica (koenig) Gmelin. Anc Sci Life. (2012)
- Koren E, et al. Supplementation with antioxidants fails to increase the total antioxidant capacity of several cell lines in culture. Biomed Pharmacother. (2008)
- Voronin MV, et al. Total antioxidant capacity of blood plasma from healthy donors receiving vitamin and mineral complex. Bull Exp Biol Med. (2004)
- Dominiak K, et al. Critical need for clinical trials: an example of a pilot human intervention trial of a mixture of natural agents protecting lymphocytes against TNF-alpha induced activation of NF-kappaB. Pharm Res. (2010)
- Everett PC, et al. Preclinical assessment of curcumin as a potential therapy for B-CLL. Am J Hematol. (2007)
- Chun KS, et al. Curcumin inhibits phorbol ester-induced expression of cyclooxygenase-2 in mouse skin through suppression of extracellular signal-regulated kinase activity and NF-kappaB activation. Carcinogenesis. (2003)
- Kunnumakkara AB, et al. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. (2007)
- Ponnurangam S, et al. Urine and serum analysis of consumed curcuminoids using an IkappaB-luciferase surrogate marker assay. In Vivo. (2010)
- Aggarwal S, et al. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol. (2006)
- Dileep KV, Tintu I, Sadasivan C. Molecular docking studies of curcumin analogs with phospholipase A2. Interdiscip Sci. (2011)
- Binion DG, et al. Vascular cell adhesion molecule-1 expression in human intestinal microvascular endothelial cells is regulated by PI 3-kinase/Akt/MAPK/NF-kappaB: inhibitory role of curcumin. Am J Physiol Gastrointest Liver Physiol. (2009)
- Kumar A, et al. Curcumin (Diferuloylmethane) inhibition of tumor necrosis factor (TNF)-mediated adhesion of monocytes to endothelial cells by suppression of cell surface expression of adhesion molecules and of nuclear factor-kappaB activation. Biochem Pharmacol. (1998)
- Jackson JK, et al. The antioxidants curcumin and quercetin inhibit inflammatory processes associated with arthritis. Inflamm Res. (2006)
- Ramadan G, Al-Kahtani MA, El-Sayed WM. Anti-inflammatory and anti-oxidant properties of Curcuma longa (turmeric) versus Zingiber officinale (ginger) rhizomes in rat adjuvant-induced arthritis. Inflammation. (2011)
- Narayanan A, et al. Curcumin Inhibits Rift Valley Fever Virus Replication in Human Cells. J Biol Chem. (2012)
- Karbalay-Doust S, Noorafshan A. Ameliorative effects of curcumin on the spermatozoon tail length, count, motility and testosterone serum level in metronidazole-treated mice. Prague Med Rep. (2011)
- Giannessi F, et al. Curcumin protects Leydig cells of mice from damage induced by chronic alcohol administration. Med Sci Monit. (2008)
- Quintans LN, Castro GD, Castro JA. Oxidation of ethanol to acetaldehyde and free radicals by rat testicular microsomes. Arch Toxicol. (2005)
- Chandra AK, et al. Effect of curcumin on chromium-induced oxidative damage in male reproductive system. Environ Toxicol Pharmacol. (2007)
- Aktas C, et al. Anti-apoptotic effects of curcumin on cadmium-induced apoptosis in rat testes. Toxicol Ind Health. (2012)
- Murphy CJ, et al. Reproductive effects of a pegylated curcumin. Reprod Toxicol. (2012)
- White EL, et al. Screening of potential cancer preventing chemicals as aromatase inhibitors in an in vitro assay. Anticancer Res. (1999)
- Valentine SP, et al. Curcumin modulates drug metabolizing enzymes in the female Swiss Webster mouse. Life Sci. (2006)
- Folwarczna J, Zych M, Trzeciak HI. Effects of curcumin on the skeletal system in rats. Pharmacol Rep. (2010)
- Bachmeier BE, et al. Reference profile correlation reveals estrogen-like trancriptional activity of Curcumin. Cell Physiol Biochem. (2010)
- Singh M, Singh N. Curcumin counteracts the proliferative effect of estradiol and induces apoptosis in cervical cancer cells. Mol Cell Biochem. (2011)
- Kinoshita A, et al. Carcinogenicity of dimethylarsinic acid in Ogg1-deficient mice. Cancer Sci. (2007)
- Biswas J, et al. Curcumin protects DNA damage in a chronically arsenic-exposed population of West Bengal. Hum Exp Toxicol. (2010)
- Mukundan MA, et al. Effect of turmeric and curcumin on BP-DNA adducts. Carcinogenesis. (1993)
- Sharma RA, et al. Effects of dietary curcumin on glutathione S-transferase and malondialdehyde-DNA adducts in rat liver and colon mucosa: relationship with drug levels. Clin Cancer Res. (2001)
- Bhattacharyya S, et al. Tumor-induced oxidative stress perturbs nuclear factor-kappaB activity-augmenting tumor necrosis factor-alpha-mediated T-cell death: protection by curcumin. Cancer Res. (2007)
- Deeb D, et al. Curcumin sensitizes prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L by inhibiting nuclear factor-kappaB through suppression of IkappaBalpha phosphorylation. Mol Cancer Ther. (2004)
- Deeb D, et al. Curcumin {1,7-bis(4-hydroxy-3-methoxyphenyl)-1-6-heptadine-3,5-dione; C21H20O6} sensitizes human prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L-induced apoptosis by suppressing nuclear factor-kappaB via inhibition of the prosurvival Akt signaling pathway. J Pharmacol Exp Ther. (2007)
- Song WB, et al. Curcumin protects intestinal mucosal barrier function of rat enteritis via activation of MKP-1 and attenuation of p38 and NF-κB activation. PLoS One. (2010)
- Kim YS, et al. Curcumin attenuates inflammatory responses of TNF-alpha-stimulated human endothelial cells. J Cardiovasc Pharmacol. (2007)
- Camacho-Barquero L, et al. Curcumin, a Curcuma longa constituent, acts on MAPK p38 pathway modulating COX-2 and iNOS expression in chronic experimental colitis. Int Immunopharmacol. (2007)
- Curcumin Inhibits Prostate Cancer Metastasis in vivo by Targeting the Inflammatory Cytokines CXCL1 and -2
- Lionaki E, Markaki M, Tavernarakis N. Autophagy and ageing: insights from invertebrate model organisms. Ageing Res Rev. (2013)
- Markaki M, Tavernarakis N. The role of autophagy in genetic pathways influencing ageing. Biogerontology. (2011)
- Pallauf K, Rimbach G. Autophagy, polyphenols and healthy ageing. Ageing Res Rev. (2013)
- Wu JC, et al. Tetrahydrocurcumin, a major metabolite of curcumin, induced autophagic cell death through coordinative modulation of PI3K/Akt-mTOR and MAPK signaling pathways in human leukemia HL-60 cells. Mol Nutr Food Res. (2011)
- Aoki H, et al. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: role of Akt and extracellular signal-regulated kinase signaling pathways. Mol Pharmacol. (2007)
- Li B, et al. Curcumin Induces Cross-Regulation Between Autophagy and Apoptosis in Uterine Leiomyosarcoma Cells. Int J Gynecol Cancer. (2013)
- Kim JY, et al. Curcumin-induced autophagy contributes to the decreased survival of oral cancer cells. Arch Oral Biol. (2012)
- Liao VH, et al. Curcumin-mediated lifespan extension in Caenorhabditis elegans. Mech Ageing Dev. (2011)
- Zhuang W, et al. Curcumin promotes differentiation of glioma-initiating cells by inducing autophagy. Cancer Sci. (2012)
- Zanotto-Filho A, et al. Curcumin-loaded lipid-core nanocapsules as a strategy to improve pharmacological efficacy of curcumin in glioma treatment. Eur J Pharm Biopharm. (2012)
- Daido S, et al. Pivotal role of the cell death factor BNIP3 in ceramide-induced autophagic cell death in malignant glioma cells. Cancer Res. (2004)
- Inhibition of the DNA-dependent protein kinase catalytic subunit radiosensitizes malignant glioma cells by inducing autophagy
- Jiang TF, et al. Curcumin Ameliorates the Neurodegenerative Pathology in A53T α-synuclein Cell Model of Parkinson's Disease Through the Downregulation of mTOR/p70S6K Signaling and the Recovery of Macroautophagy. J Neuroimmune Pharmacol. (2013)
- Soh JW, et al. Curcumin is an early-acting stage-specific inducer of extended functional longevity in Drosophila. Exp Gerontol. (2013)
- Lifespan Extension by the Antioxidant Curcumin in Drosophila Melanogaster
- Lee KS, et al. Curcumin extends life span, improves health span, and modulates the expression of age-associated aging genes in Drosophila melanogaster. Rejuvenation Res. (2010)
- Kitani K, Osawa T, Yokozawa T. The effects of tetrahydrocurcumin and green tea polyphenol on the survival of male C57BL/6 mice. Biogerontology. (2007)
- Kitani K, Yokozawa T, Osawa T. Interventions in aging and age-associated pathologies by means of nutritional approaches. Ann N Y Acad Sci. (2004)
- Spindler S, et al. Influence on longevity of blueberry, cinnamon, green and black tea, pomegranate, sesame, curcumin, morin, Pycnogenol, quercetin and taxifolin fed isocalorically to long-lived, outcrossed mice. Rejuvenation Res. (2013)
- Strong R, et al. Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid, and medium-chain triglyceride oil on life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. (2013)
- Food Intake, Water Intake, and Drinking Spout Side Preference of 28 Mouse Strains
- Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Healthy Adult Volunteers
- Hanai H, et al. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. (2006)
- Holt PR, Katz S, Kirshoff R. Curcumin therapy in inflammatory bowel disease: a pilot study. Dig Dis Sci. (2005)
- Um MY, et al. Curcumin Attenuates Diet-Induced Hepatic Steatosis by Activating AMPK. Basic Clin Pharmacol Toxicol. (2013)
- Khajehdehi P, et al. Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-β and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: a randomized, double-blind and placebo-controlled study. Scand J Urol Nephrol. (2011)
- Zhong F, et al. Curcumin attenuates lipopolysaccharide-induced renal inflammation. Biol Pharm Bull. (2011)
- Ghosh SS, et al. Curcumin ameliorates renal failure in 5/6 nephrectomized rats: role of inflammation. Am J Physiol Renal Physiol. (2009)
- Hill-Kapturczak N, et al. Mechanism of heme oxygenase-1 gene induction by curcumin in human renal proximal tubule cells. Am J Physiol Renal Physiol. (2001)
- Gaedeke J, Noble NA, Border WA. Curcumin blocks fibrosis in anti-Thy 1 glomerulonephritis through up-regulation of heme oxygenase 1. Kidney Int. (2005)
- Sehgal A, et al. Synergistic effects of piperine and curcumin in modulating benzo(a)pyrene induced redox imbalance in mice lungs. Toxicol Mech Methods. (2012)
- Sehgal A, et al. Piperine as an adjuvant increases the efficacy of curcumin in mitigating benzo(a)pyrene toxicity. Hum Exp Toxicol. (2012)
- Sehgal A, et al. Combined effects of curcumin and piperine in ameliorating benzo(a)pyrene induced DNA damage. Food Chem Toxicol. (2011)
- Hlavačková L, et al. Spice up the hypertension diet - curcumin and piperine prevent remodeling of aorta in experimental L-NAME induced hypertension. Nutr Metab (Lond). (2011)
- Murakami A, et al. Curcumin combined with turmerones, essential oil components of turmeric, abolishes inflammation-associated mouse colon carcinogenesis. Biofactors. (2013)
- Bhagavathula N, et al. A combination of curcumin and ginger extract improves abrasion wound healing in corticosteroid-impaired hairless rat skin. Wound Repair Regen. (2009)
- Madkor HR, Mansour SW, Ramadan G. Modulatory effects of garlic, ginger, turmeric and their mixture on hyperglycaemia, dyslipidaemia and oxidative stress in streptozotocin-nicotinamide diabetic rats. Br J Nutr. (2011)
- Ide H, et al. Combined inhibitory effects of soy isoflavones and curcumin on the production of prostate-specific antigen. Prostate. (2010)
- Altenburg JD, et al. A synergistic antiproliferation effect of curcumin and docosahexaenoic acid in SK-BR-3 breast cancer cells: unique signaling not explained by the effects of either compound alone. BMC Cancer. (2011)
- Saw CL, Huang Y, Kong AN. Synergistic anti-inflammatory effects of low doses of curcumin in combination with polyunsaturated fatty acids: docosahexaenoic acid or eicosapentaenoic acid. Biochem Pharmacol. (2010)
- Baum L, Ng A. Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer's disease animal models. J Alzheimers Dis. (2004)
- Ishihara M, Sakagami H. Re-evaluation of cytotoxicity and iron chelation activity of three beta-diketones by semiempirical molecular orbital method. In Vivo. (2005)
- Jiao Y1, et al. Curcumin, a cancer chemopreventive and chemotherapeutic agent, is a biologically active iron chelator. Blood. (2009)
- Tuntipopipat S, et al. Chili, but not turmeric, inhibits iron absorption in young women from an iron-fortified composite meal. J Nutr. (2006)
- Padhye S, et al. Emerging role of Garcinol, the antioxidant chalcone from Garcinia indica Choisy and its synthetic analogs. J Hematol Oncol. (2009)
- Parasramka MA, Gupta SV. Synergistic effect of garcinol and curcumin on antiproliferative and apoptotic activity in pancreatic cancer cells. J Oncol. (2012)
- Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. (2003)
- Baum L, et al. Curcumin effects on blood lipid profile in a 6-month human study. Pharmacol Res. (2007)
- Kuptniratsaikul V, et al. Efficacy and safety of Curcuma domestica extracts in patients with knee osteoarthritis. J Altern Complement Med. (2009)
- Wickenberg J, Ingemansson SL, Hlebowicz J. Effects of Curcuma longa (turmeric) on postprandial plasma glucose and insulin in healthy subjects. Nutr J. (2010)
- Appendino G, et al. Potential role of curcumin phytosome (Meriva) in controlling the evolution of diabetic microangiopathy. A pilot study. Panminerva Med. (2011)
- Chainani-Wu N, et al. High-dose curcuminoids are efficacious in the reduction in symptoms and signs of oral lichen planus. J Am Acad Dermatol. (2012)
- Koosirirat C, et al. Investigation of the anti-inflammatory effect of Curcuma longa in Helicobacter pylori-infected patients. Int Immunopharmacol. (2010)
- Kalpravidh RW, et al. Improvement in oxidative stress and antioxidant parameters in beta-thalassemia/Hb E patients treated with curcuminoids. Clin Biochem. (2010)
- Alwi I, et al. The effect of curcumin on lipid level in patients with acute coronary syndrome. Acta Med Indones. (2008)
- Durgaprasad S, et al. A pilot study of the antioxidant effect of curcumin in tropical pancreatitis. Indian J Med Res. (2005)
- Carroll RE, et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev Res (Phila). (2011)
- He ZY, et al. Upregulation of p53 expression in patients with colorectal cancer by administration of curcumin. Cancer Invest. (2011)
- Shimouchi A, et al. Effect of dietary turmeric on breath hydrogen. Dig Dis Sci. (2009)
- Sanmukhani J1, et al. Efficacy and Safety of Curcumin in Major Depressive Disorder: A Randomized Controlled Trial. Phytother Res. (2013)